Introduction: Since FDA approval in 2017, chimeric antigen receptor T-cell (CAR-T) therapy has become an integral part of the treatment algorithm for diffuse large B-cell lymphoma (DLBCL). Despite CD19-modified CAR-T effectiveness in multiple prospective clinical trials, with the transition to a commercial product at an authorized treatment center (ATC) it became apparent that multiple steps additional steps were needed before apheresis for a commercial CAR-T cell product. The workflow for an ATC namely during the B2V time appeared to be significantly different than the clinical trial experience. There remains a dearth of data that evaluates the impact of the B2V time. Intent to pursue CAR-T therapy in the commercial environment has two time periods: Brain-to-Vain (B2V) and Vein-to-Vein (V2V). The B2V time encapsulates referral and evaluation for CAR-T, purchase authorization, when needed negotiation of a single case agreement (SCA), and slot assignment for collection of a cellular product. The time between apheresis and cellular product infusion is known as the V2V period, which is product specific and established from prospective clinical trials. We identified that patients with private insurance or Medicare with managed plans at our institution often require a SCA. A SCA is a contract between the insurance company and the ATC that allows the patient to receive therapy using their in-network benefits. We hypothesized there would be a discrepancy in the B2V time between patients who required a SCA and those who did not. We further hypothesized that patients who did not require a SCA would experience significantly improved treatment outcomes, including a higher percentage achieving the best response of complete response (CR) with a superior prolonged overall survival (OS).
Methods: To test these hypotheses, we performed a single-center retrospective study of consecutively evaluated patients with DLBCL who had at least two prior lines of therapy and intended to receive CAR-T therapy with a single CAR-T product at the University of Nebraska from 2018-2022. Patient and disease characteristics were collected. Intent to CAR-T (iCAR-T) was defined as the date of consultation at the ATC to date of apheresis of the product. Cellular therapy intent quotient (CTIQ) was defined as the number of infused with a CAR-T product versus those who intended to go to CAR-T. Those patients who intended to go to CAR-T but did not proceed to apheresis or product infusion were included in the calculation of CTIQ and OS.
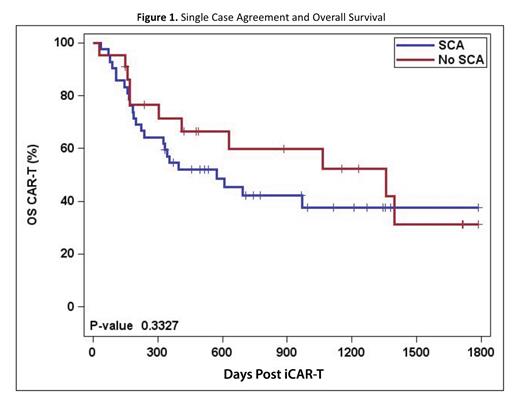
Results: Of 64 patients who intended to receive CAR-T therapy, 42 required a SCA (66%). Most (39/42) of the patients requiring a SCA had private insurance. All (22/22) of the patients in the non-SCA cohort had public insurance. The was no difference between cohorts based on gender, disease characteristics, distance traveled to ATC, and prior lines of therapy. As expected, the non-SCA cohort was significantly older than the SCA cohort, with mean ages of 69 and 55 respectively (p=0.0001). The CTIQ for the entire group was 94% (60/64). Of the 42 patients who required a SCA before apheresis, 39 (CTIQ– 92.8%) underwent apheresis. Of the 22 patients who did not require a SCA, 21 (CTIQ–95.5%) underwent apheresis. Of the patients who were apheresed, the B2V time was significantly longer for the SCA (42 days; 15-92) compared to the non-SCA cohorts (24 days; range 7-78)(p=0.0005). The average V2V times for the SCA and non-SCA groups were comparable at 29 and 31 days, respectively. All patients who underwent apheresis had their CAR-T cells infused. No significant difference in best response of CR or OS was observed. The best response of CR for the SCA and non-SCA groups was 46% and 57%, respectively (p=0.417). The median OS post-iCAR-T for the SCA group was 19 months, and the median OS for the non-SCA group was 45.2 months (p=0.3327).
Conclusion: Patients who require a SCA experienced a significantly longer time to CAR-T apheresis compared to those not requiring a SCA. The longer B2V time acknowledges a period that is often unaccounted for in other trials. Our assessment was limited due to small cohort size and implementation of bridging therapies (results to be presented). Further investigation comparing the CTIQ and B2V time to outcomes with commercial products is warranted to ensure patients are receiving equitable access regardless of insurance type.
Disclosures
Armitage:Cardiff Oncology: Membership on an entity's Board of Directors or advisory committees. Vose:Eli Lilly and Company; Epizyme, Kite, Loxo, Novartis: Research Funding; AbbVie, MEI Pharma: Consultancy. Lunning:AbbVie: Consultancy, Honoraria; Acrotech: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Caribou: Consultancy, Honoraria; CRISPR: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; EUSA: Consultancy, Honoraria; Fate Therapeutics: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; GenMab: Consultancy, Honoraria; InstilBio: Consultancy, Honoraria; Ipsen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Loxo: Consultancy, Honoraria; Miltenyi: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Nurix: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; SeaGen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Curis: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal